Developing Software as a Medical Device
Companies in the healthcare market are facing a major challenge: they want to expand their offering with digital solutions – but at the same time have to comply with a large number of regulatory requirements and laws.
Digital solutions, such as medical apps, can increase the effectiveness of treatment offerings and adherence and thus make a significant contribution to the success of treatment. However, healthcare companies that want to expand their offering must also take into account national and international legislation on data protection and data security as well as a large number of regulatory requirements for quality assurance, usability and risk management. The challenge is therefore great. Producing standards-compliant documentation alone is an almost insurmountable task for many companies, due to its structure and formalism.
TWT Health is certified in accordance with DIN EN ISO 13485 and ISO 27001 and is therefore qualified and approved for the development and operation of software and apps in the healthcare sector.
Whitepaper (in German language): "Medizinische Apps – smarte Helfer oder komplexe Medizinprodukte?"
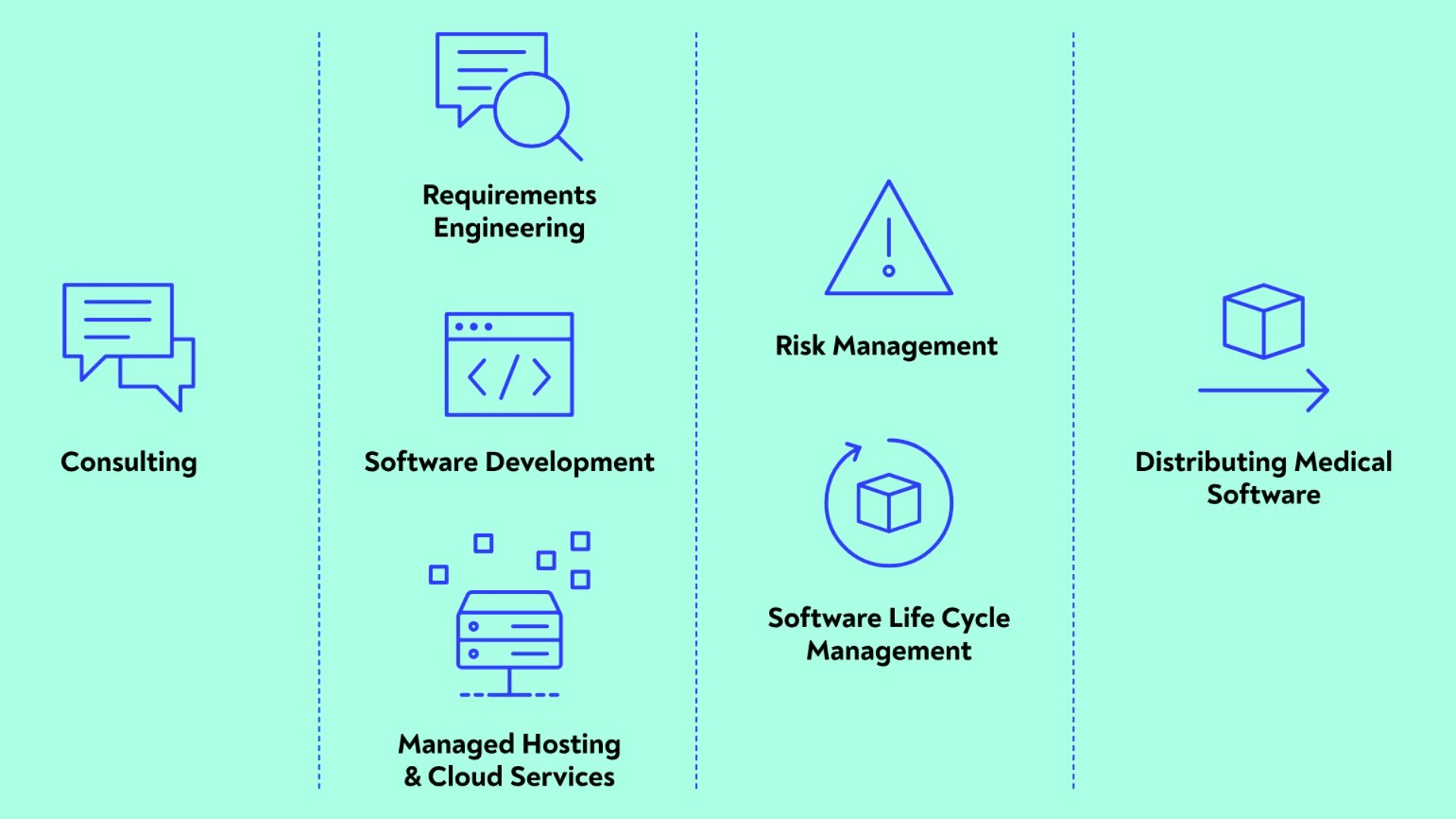
Developing software as a medical device
We plan, design and implement software for you in strict accordance with the EN IEC 62304 software life cycle and the EN IEC 62366 usability requirements. In doing so, we document all interim and final results in our quality management system, meeting the requirements of EN ISO 13485. Risk management in accordance with EN ISO 14971, where we identify unknown risk, assess the identified risk and implement appropriate control measures, is applied to each phase of development.
Standards-compliant requirements engineering
Together with our customers we gather the requirements for the software according to the normative specifications of EN IEC 62366 in Europe and the USA. These are prerequisites for the implementation of the software life cycle (EN IEC 62304) and quality assurance (EN ISO 13485).
Standards-compliant architecture and design planning
We draft the architecture and design of your software and provide you with all the necessary documents, such as the software life-cycle file, the software requirements specification (SRS) and the software verification and test logs, based on the requirements.
Standards-compliant risk identification, assessment and control
We check the software concept for unknown risk, assess the risk and devise control measures, in accordance with risk-management requirements (EN ISO 14971).
Measuring and implementing usability in accordance with regulatory requirements
Due to our cooperation partners, we can validate usability in accordance with EN IEC 62366 in Europe and the USA.
Standards-compliant software operation
We support you in the PMS phase and work with you to define the vigilance processes for dealing with adverse events.
Necessary documents for the medical-device software conformity assessment
We prepare all the documents required for an MDD or MDR, or an FDA CFR conformity assessment. All the documentation is produced in our electronic quality management system (eQMS), which enables us to automatically sign documents, such as the Technical Documentation, or the Device Master Record and the Design History File (FDA), and make them available in digital format. In doing so, we triple-check the conformity of the documents and store all versions in accordance with audit-trail procedures.
Let's Make Digital Health Happen!
We can take on all tasks for you, from planning and development to operation, and realize your digital health idea as your engineering partner.
Regardless of whether you already have a concrete plan or just a rough idea - talk to us! We look forward to your digital challenge.
References and download area with whitepapers & case studies (in German language) ...