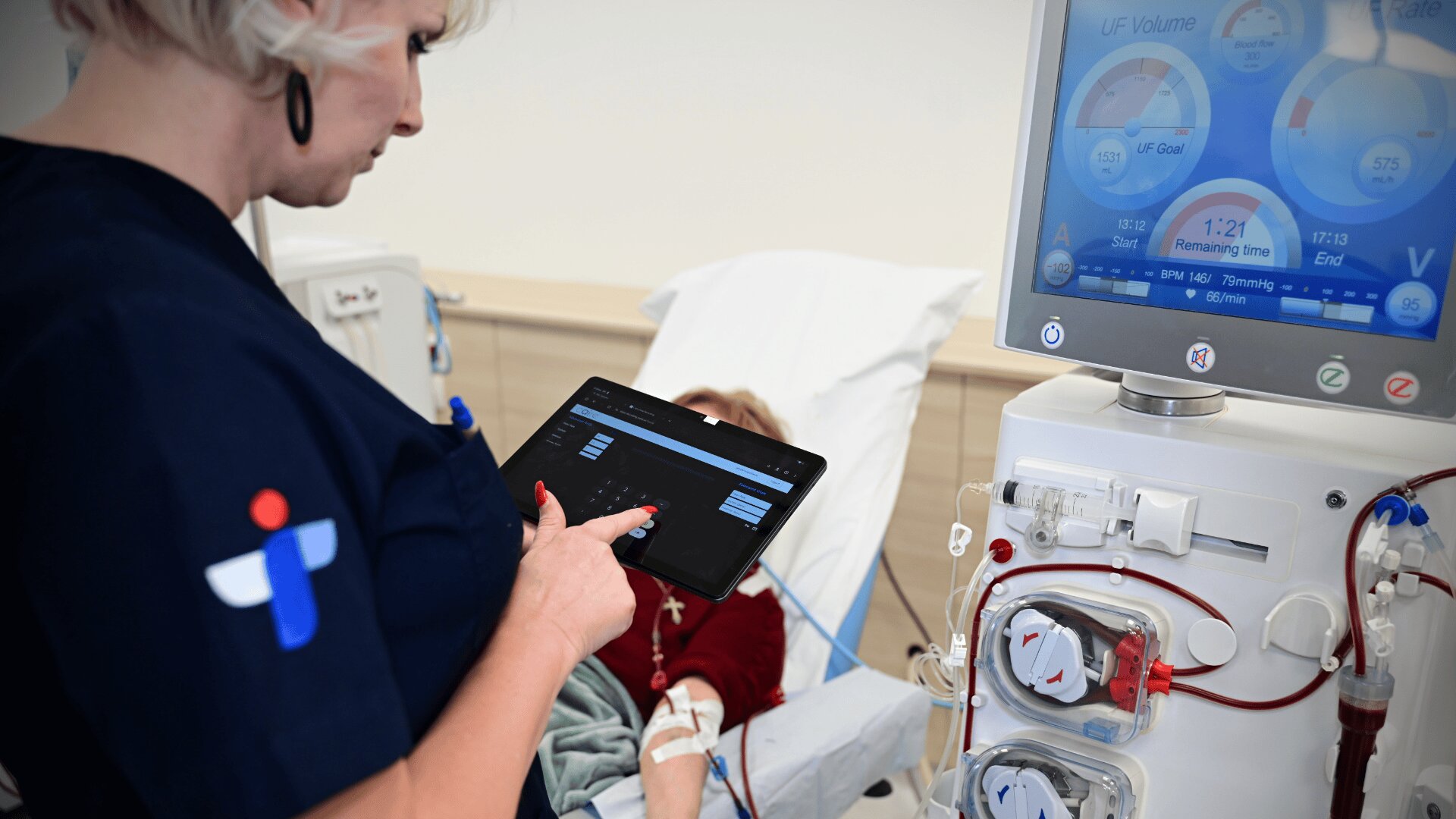
Empowering medical device startups to conquer the market
D.med Technologies guides you through the complex landscape of medical device development. In the world of medical technology startups, turning a brilliant idea into a successful medical product requires a journey full of critical decisions and technical hurdles.
At D.med Technologies, we understand the fears and uncertainties startups face on this journey.
That is why we have become their strategic ally, offering comprehensive consulting and development solutions to help them succeed.
Some of the challenges faced by startups include:
-
The complexity of the medical device development process, from research and design to regulatory approval and commercialisation, can be overwhelming for startups lacking experience in the field.
-
Startups often have limited financial and human resources, making it difficult to recruit experts in the various fields required to successfully develop a medical device.
-
Medical device regulations are complex and vary by region, which can be a significant challenge for startups seeking to market their products globally.
-
Securing the necessary funding to develop and market a medical device can be a major hurdle for start-ups.
At D.med Technologies, we have designed our services to comprehensively address these needs. We offer a full range of solutions tailored to the needs of these startups, including:
-
Development Consulting: Our team of medical device development experts provides guidance and support at all stages of the development process, from initial ideation through to regulatory approval and commercialisation.
-
Optimising device profitability: We help startups optimise the profitability of their medical devices through cost analysis, pricing strategies and efficient manufacturing solutions.
-
Medical Hardware Development: Our experienced engineers design and develop customised medical hardware that meets your specific requirements and applicable regulations.
-
Software Development: We have a team of expert software developers who create secure and reliable medical software for your devices.
-
Cybersecurity: We protect your medical device from increasingly sophisticated cyber threats through security assessments, penetration testing and integrated security solutions.
-
Verification and validation: We ensure your medical device meets all security and performance requirements through rigorous verification and validation processes.
-
Regulatory Affairs: We navigate the complex regulatory landscape for medical devices and help obtain the necessary approvals to market your product.
-
Clinical studies: We design and execute clinical studies, to evaluate the safety and efficacy of your medical device.
-
Medical/clinical expertise: Our team of doctors and clinicians brings deep experience to ensure that your medical device meets the needs of patients and healthcare professionals. We have our own state-of-the-art medical research facility, which gives you access to a clinical environment for testing and pilot studies of your medical device. This allows you to collect valuable data and obtain real-time feedback from patients and healthcare professionals, helping you to refine your device and increase its chances of success.
-
Market Access: We assist you in developing market access strategies and connecting you with the right partners and distributors to bring your medical device to the patients who need it.
- Prototyping and high precise test equipment: We provide measurement methods, development and manufacturing of customized prototypes and high precise test equipment to meet special requirements for complex medical devices and calibration services for test equipment.
Discover how we can guarantee your peace of mind.
Contact us today and find out how we can help you turn your startup into a successful business.