Introducing the "SoHo" Regulation 2024/1938
The landscape of substances of human origin (SoHO) intended for human application has evolved! With the adoption of the "SoHo Regulation 2024/1938)", a new chapter begins in ensuring safety, transparency, and innovation in medical applications.
be-on-quality GmbH
Reichenschwand,
Germany
🔍 What's new?
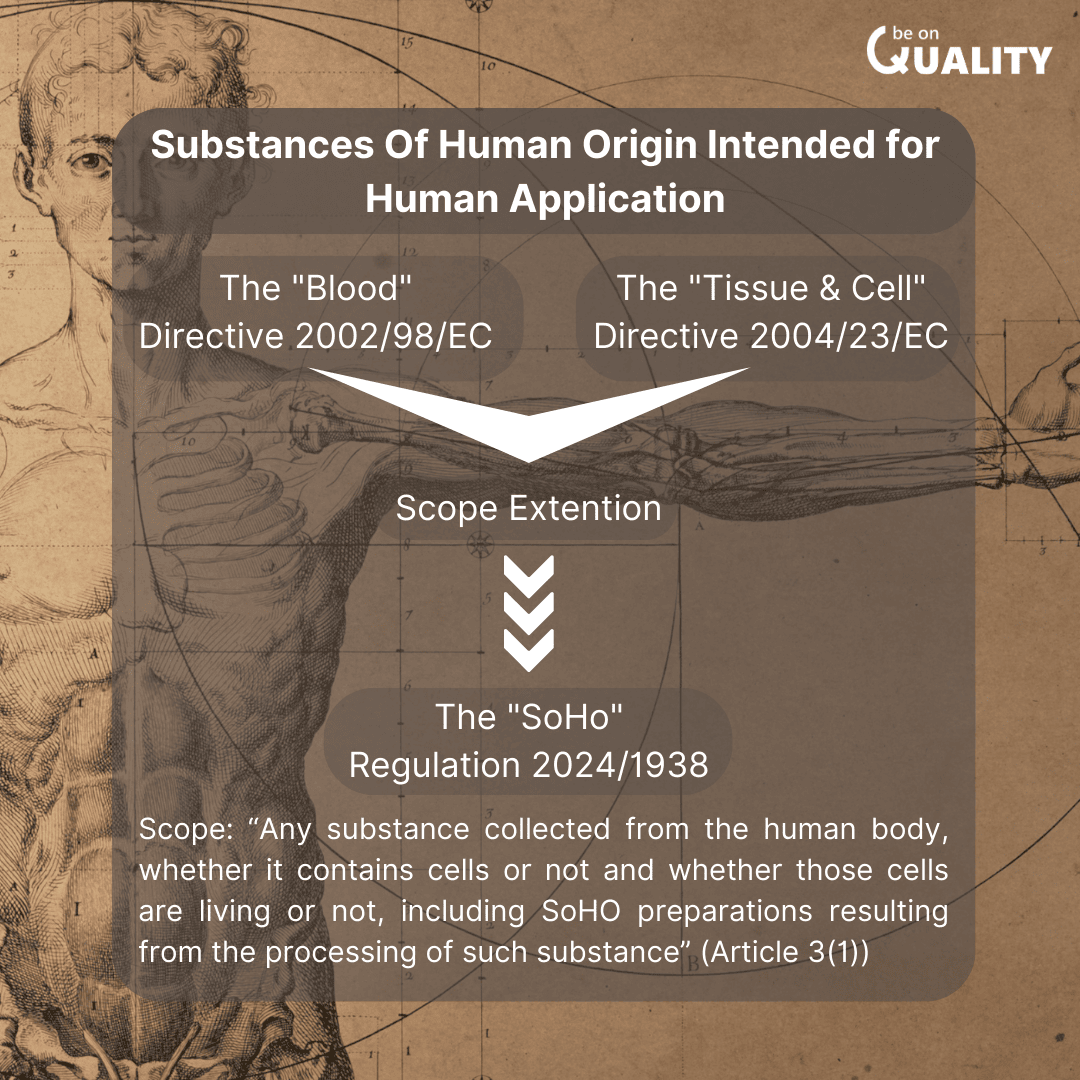
The regulation extends the scope of the existing:
- Blood Directive (2002/98/EC)
- Tissue and Cell Directive (2003/23/EC)
💡 Why does this matter?
- Increased Oversight: Strengthened framework for donor protection and product safety.
- Clarity in Definitions: Reduces ambiguity for stakeholders in healthcare, research, and industry.
- Broad Impact: Impacts a wide range of human-origin materials, aligning with modern medical and scientific advancements.
📢 Next Steps:
The new Regulation will apply as from 7 August 2027, 3 years after its publication and entry into force, with an extra year for certain provisions.