The Biomedical Technology Center at Europe's largest textile research center researches and develops from the raw material through all innovation steps of the textile chain to the production-ready product. Certified according to ISO 13485, we are your partner in fiber based medical technology.
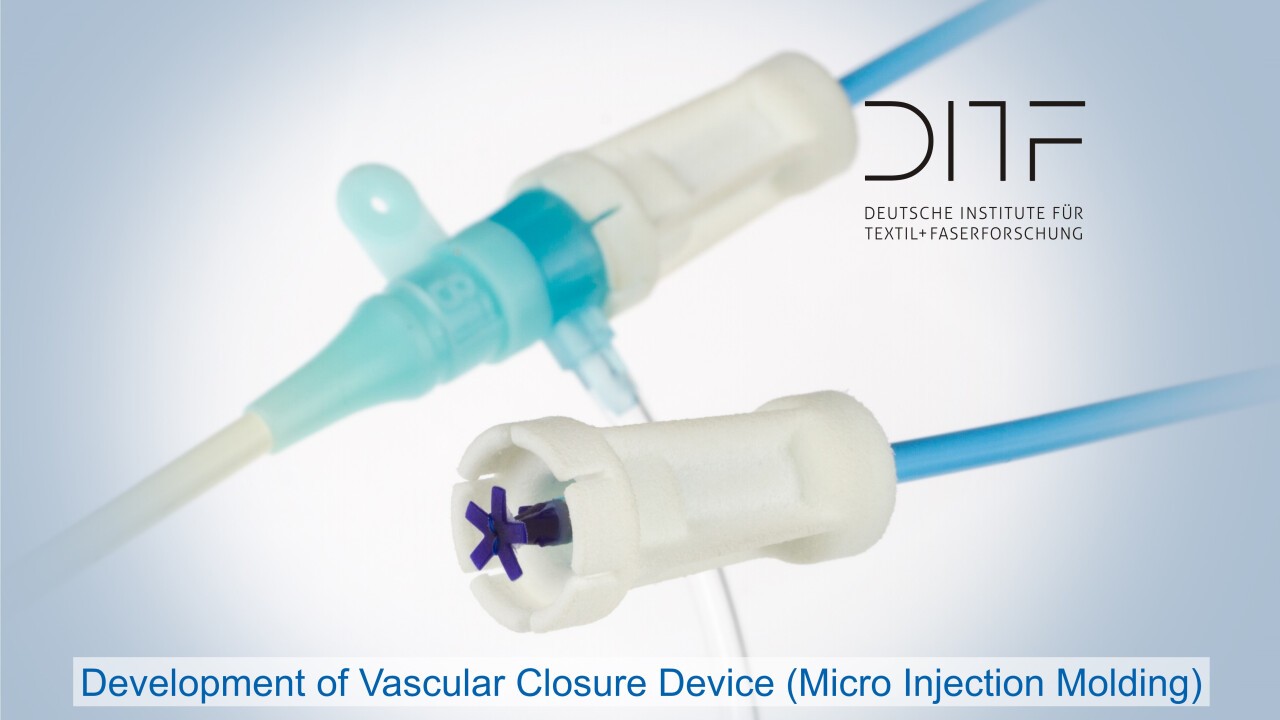
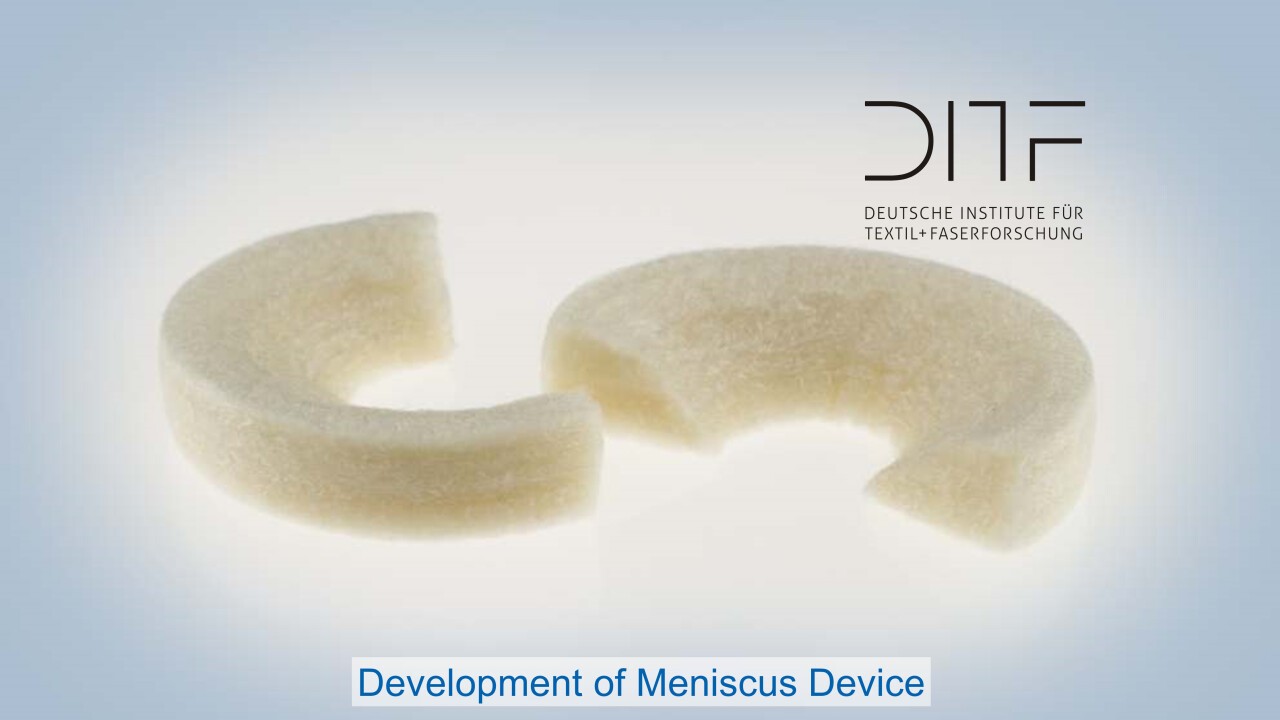
One focus is the development of novel biomaterials based on polymers, which are also combined with ceramic materials depending on requirements. The starting materials are processed into resorbable or biostable implants and into carrier materials for cell colonization in regenerative medicine. DITF developments in these areas support the paradigm shift towards personalized medicine: Therapeutic products and procedures are designed in such a way that they can be tailored to the very individual needs of patients. In addition to textile technologies and thermoplastic processing through injection molding and extrusion, the focus is also on additive manufacturing processes.
Sensory and actuator medical products and drug delivery systems are just as much a part of the development portfolio as the entire range of classic medical textiles, from wound dressings and hospital textiles to compression textiles. Therapy support systems are also being made more flexible so that they can be adapted to individual patients or patient groups in line with the trend towards personalized medicine.
The DITF maintain research and development partnerships with medical technology companies, university hospitals and research institutes worldwide. The in-house interdisciplinary team includes scientists from medical, process and textile engineering, biology and chemistry.
Topics:
- Medical fibers and nonwovens
- Antibacterial and antiviral finishes
- Therapeutic textile products
- Drug delivery systems
- Theranostic systems
- Additive processes for individualized medicine
- Textile-based sensors and actuators, coupled with artificial intelligence (AI)
- Clinical and ambulatory health monitoring
- Personal protective equipment
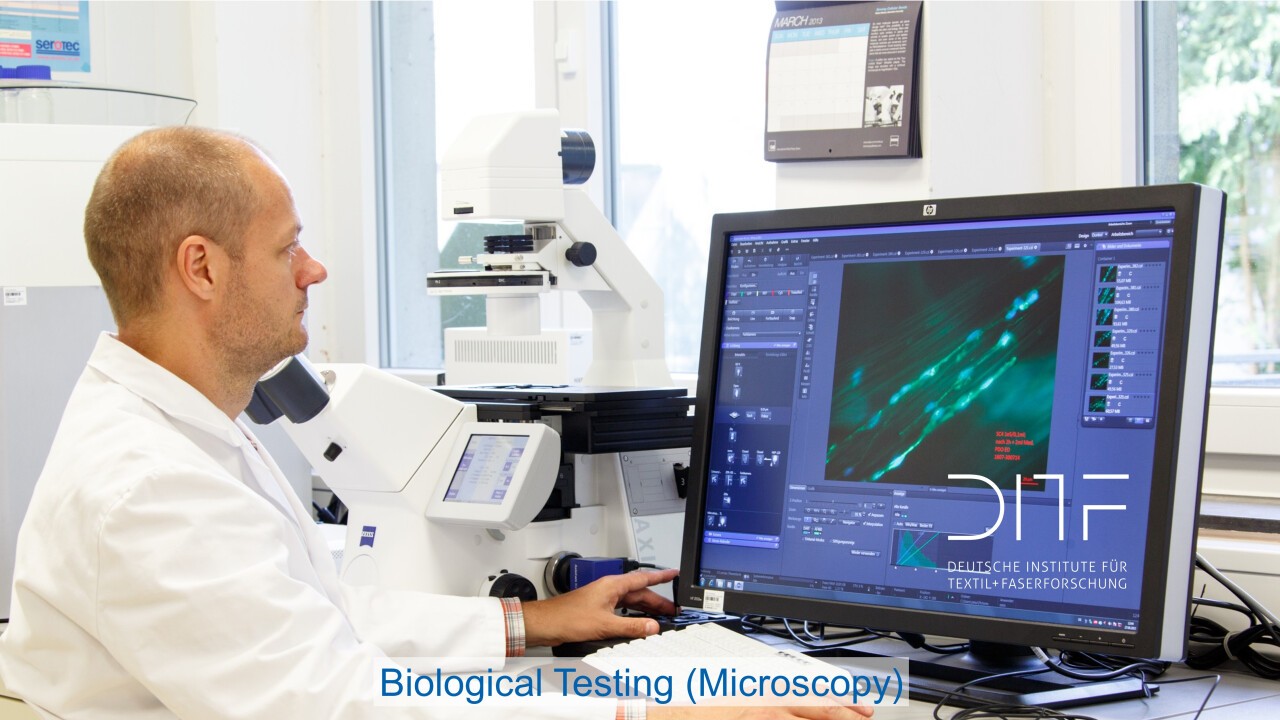
Testing laboratory biology
In vitro cytotoxicity tests according to the "biological evaluation of medical devices" as well as microbiological tests such as e.g. biocontamination control, determination of bioburden and various tests for antibacterial effects.
In addition, the biological test laboratory offers the testing of textiles that are mainly worn close to the skin for body compatibility in accordance with the criteria of the test seal "MEDICALLY TESTED - TESTED FOR TOXINS" of the Fördergemeinschaft Körperverträgliche Textilien e. V. (FKT). We can also advise you in the production of body-compatible textiles.
Service overview (selection):
- Medical device testing for in vitro cytotoxicity according to DIN EN ISO 10993-5
- Hygiene monitoring: biocontamination of air, compressed gases and surfaces
- Determination of the population of microorganisms on products (bioburden)
- Determination of antibacterial activity
- Testing for resistance to dry microbial penetration
- Body compatibility test for textiles worn close to the skin
- Wearing simulation and substance transfer
- cytotoxicity test with fibroblasts
- Skin irritation test with human keratinocytes
- further tests on request