The ABCs of Product Lifecycle Reporting under the MDR – How to Stay on Track!
The requirements of the MDR for the documentation of medical devices are complex. However, with the right strategy, it’s not rocket science to efficiently implement all phases – from clinical evaluation to post-market surveillance.
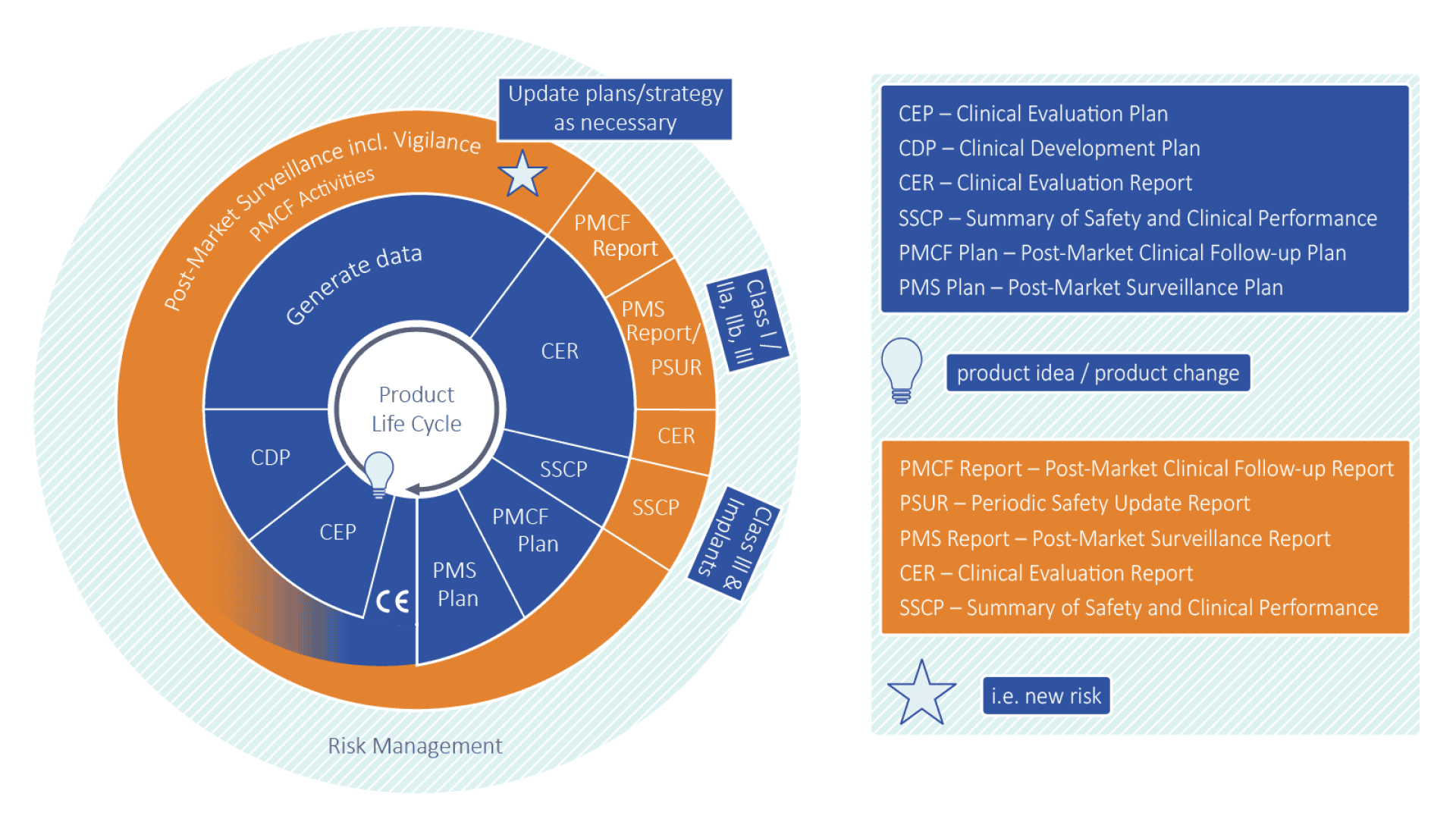
A key success factor in implementing the MDR is a well-coordinated strategy for product lifecycle reporting. This strategy should ensure that all relevant processes are efficiently interconnected, from clinical evaluation to the post-market surveillance (PMS) system. Only through close integration of the different phases can risks be identified in time and appropriate actions be taken.
The challenge lies in the fact that the requirements apply not only during development and market introduction but must also be continuously adjusted and updated throughout the entire lifecycle of a product. Forward planning and regular updates of reports are crucial to meet the requirements at all times and remain flexible in response to changes.
With a clear strategy and a well-structured process, manufacturers can efficiently meet all MDR requirements and ensure their products remain safe and effective even after market introduction.
Want to learn how to optimally manage your product lifecycle reporting under the MDR? Our blog post explains the process step by step.