The Perfect Combination: RIM System and Regulatory Services
Implementing a RIM system? Even the best software isn’t enough on its own. Rely on the perfect combination of a technological system and human expertise.
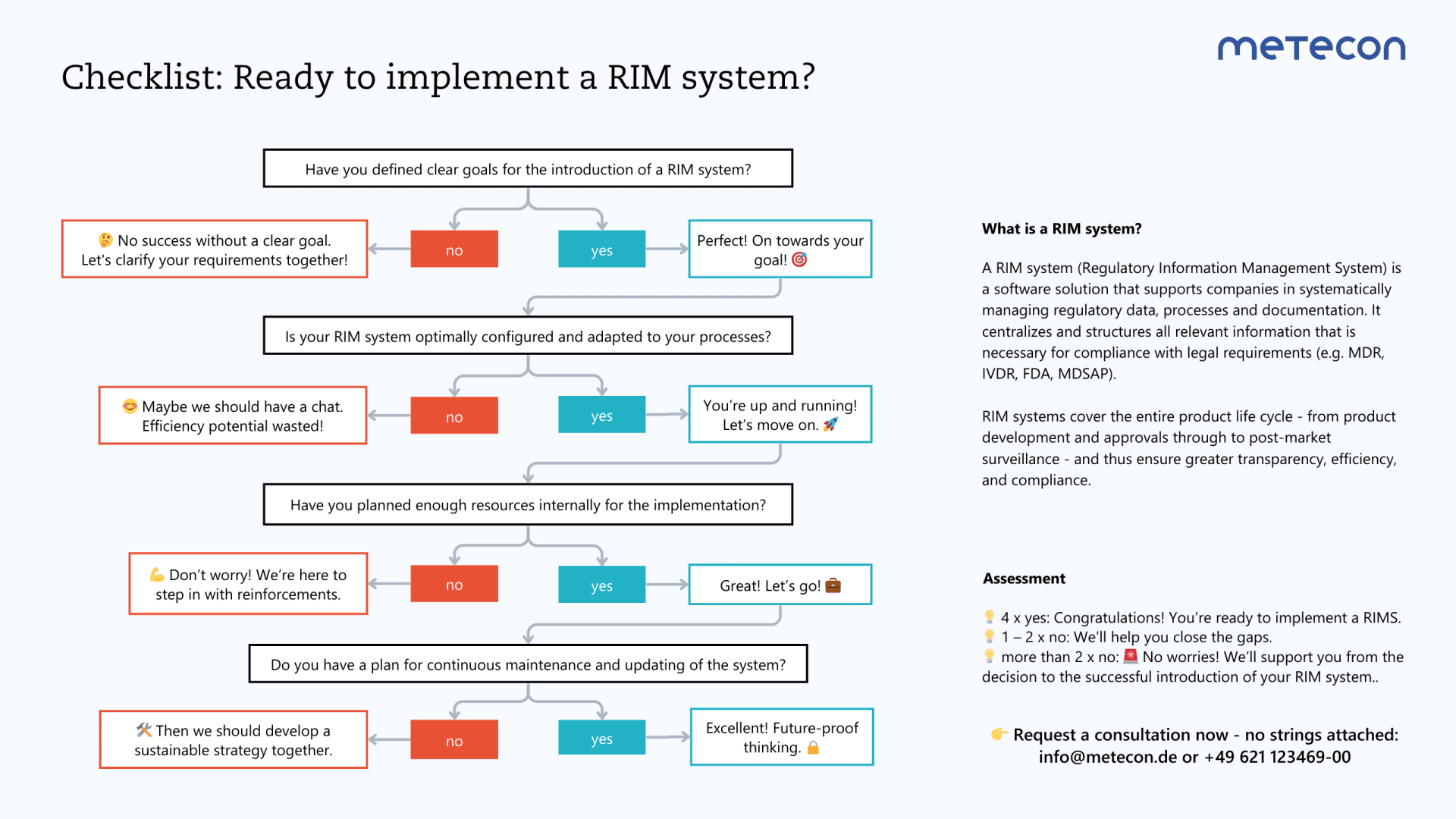
Regulatory requirements pose significant challenges for manufacturers of medical devices and in-vitro diagnostics. A RIM system (Regulatory Information Management System) brings structure and efficiency, but it’s not enough on its own. Only when combined with the strategic expertise of an experienced regulatory partner does it reach its full potential.
We offer more than just technical support:
- Data Quality and Best Practices: Your documentation is not only accurately maintained but also optimally aligned with your processes.
- Strategic Decisions: We interpret complex requirements, minimize compliance risks, and support you in critical processes such as audits or product approvals.
- Flexibility and Transparency: With our expertise, you’ll be well-prepared for dynamic demands—whether through targeted resource utilization or continuous process optimization.
Discover how the synergy of a RIM system and human expertise can make your regulatory strategy future-proof. Learn more in our blog post!