Standards-compliant FPGA development for medical technology
For many people, the use of FPGAs in the development of medical products is technically a rather blank page compared to the more familiar hardware or software development. We support our customers in integrating the advantages of FPGAs into their development efficiently and in compliance with standa
What are the advantages of using FPGAs?
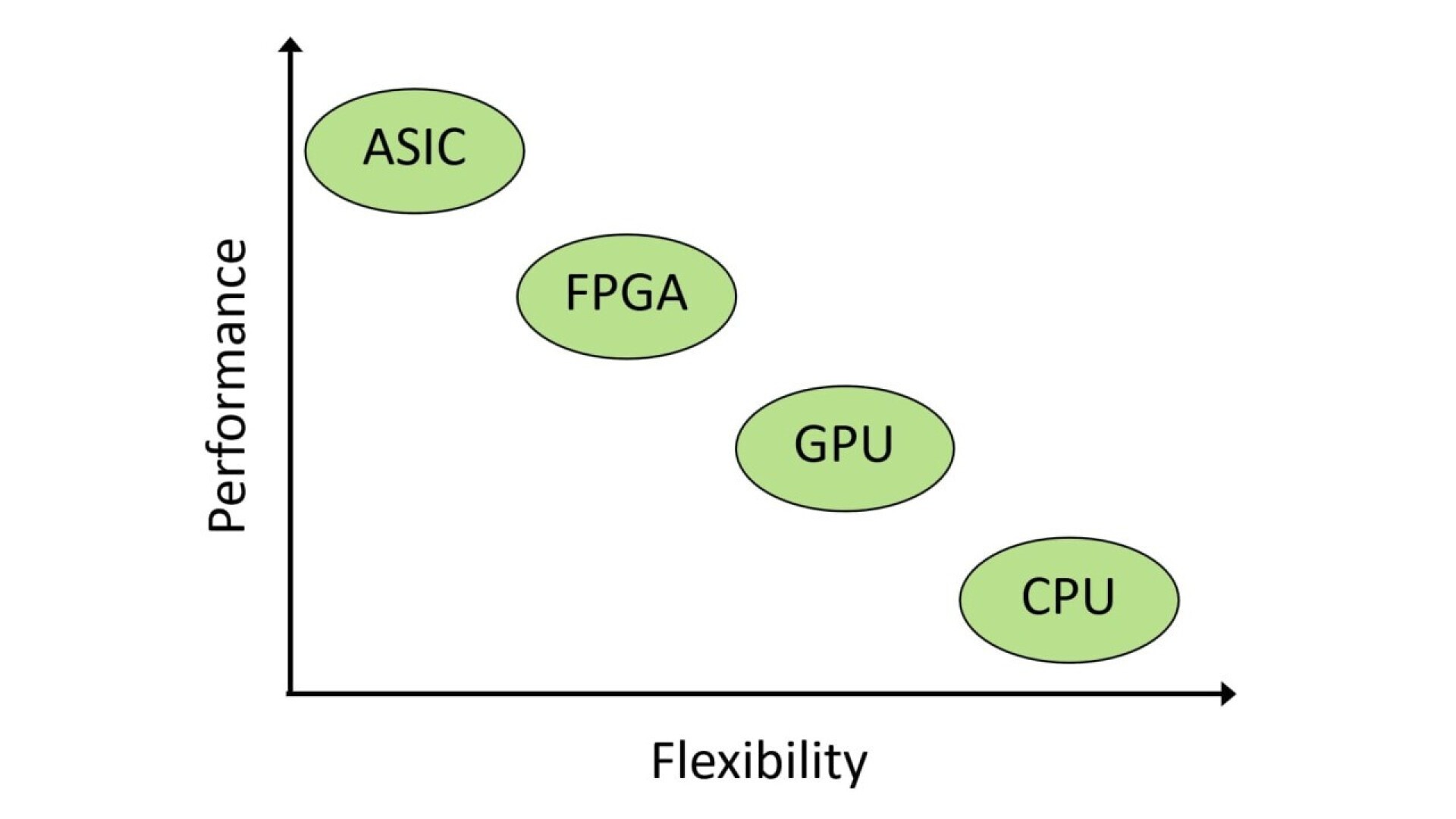
A major advantage of using FPGAs (Field Programmable Gate Arrays) is that the performance benefits of dedicated hardware solutions can be combined with the advantages of software solutions in terms of their flexibility. It is also important that when programming an FPGA, the time and logic sequences are not programmed as with conventional software, but the functional structure of the component is defined in a description language such as VHDL.
When are FPGAs used?
The use of FPGAs is particularly suitable for systems with hard real-time requirements or safety-relevant aspects. In an FPGA, a critical signal path can be mapped directly in the hardware and can be executed in parallel with the actual functionality of the design. Because data can be processed in the FPGA in a highly parallel manner, they are also predestined for processing large amounts of data, such as in image processing applications.
What are the normative requirements for FPGA development?
Precisely because of the middle ground between hardware and software, there are often different views on the classification of FPGA development with regard to normative requirements. In our opinion, however, the answer to the classification is clear: In regulatory terms, FPGA development is expressly subject to the same requirements as hardware development.
FPGA development: Our approach at solectrix GmbH
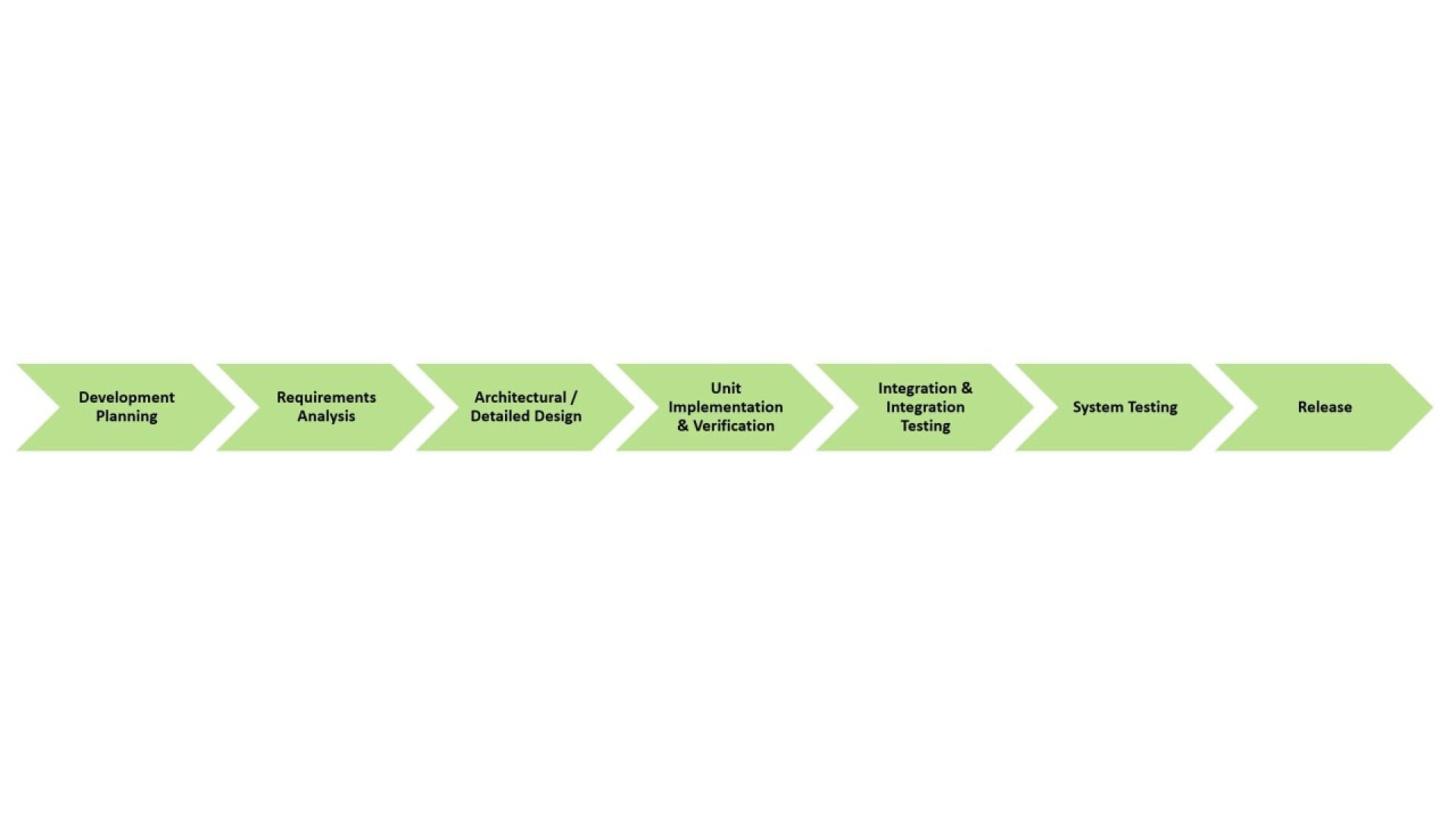
Based on the requirements and phases from chapter 5 of EN 62304, the following procedure has been established within solectrix GmbH for FPGA development:
- Creation of a development plan
- Definition of coding guidelines. The guidelines are checked in the development project using static code analyses
- Specification of extended rules to be observed when coding in VHDL to increase the quality of the design to be created
- Creation of a design specification
- Specification of the functional requirements for the FPGA
- Identification and description of the required function blocks and interfaces
- Description of all external interfaces of the device
- Definition of driver strengths, level standards and timing behavior
- Implementation based on the previously defined principles
- Implementation is based on the design specification
- Explicit documents on the architecture or a detailed design, as known from software development, are covered by the creation of the function description in VHDL and the description of the function blocks
- The planned verification environment is set up at this point together with the development
- Verification planning
- Description of the verification environment for the FPGA project
- Definition of all tools and frameworks to be used for verification, such as
- Simulations or timing analyses
- Consideration of functional, code and branch coverage
- In contrast to software, validation of the tool chain is generally not necessary, as he desired FPGA functionality is verified in the (sub)system
- Release process
- The procedure for releasing a release does not differ significantly from the procedure within software development
- The output checked according to the verification plan is delivered as a netlist or bitstream as input for the HW/SW integration. Verification at integration level can only take place on the target system